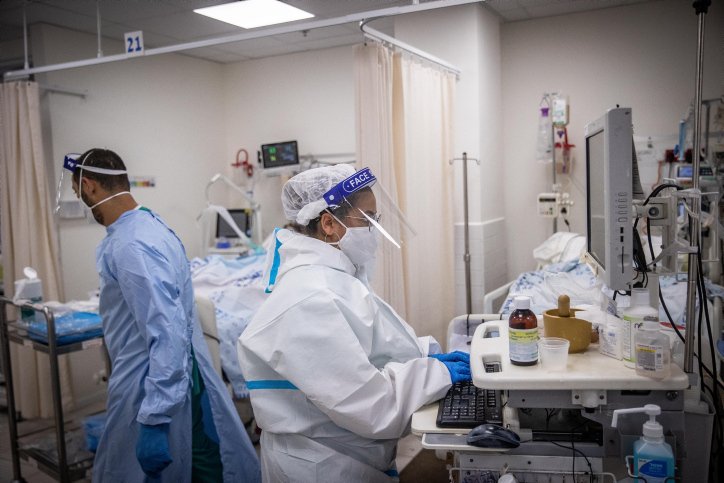
The Israel Ministry of Health announced on Wednesday that it would purchase a quota of the potentially life-saving drug “REGEN-COV,” which could be used to treat mild-to-moderate COVID-19 patients. According to the Israeli media, this drug was not offered to most patients due to high costs. There was talk of downright withholding it by the health authorities in Israel. The ministry’s decision to purchase the drug can therefore be seen as a complete policy about-face.
- How did this U-turn come about?
- Why is an apparently effective drug knowingly withheld from far too many patients for so long?
The public is right to ask these questions.
Especially since there is already a drug developed in Israel, the effectiveness of which has been shown to be very high in internal hospital studies. In April, for example, Israeli public broadcaster KAN reported that researchers at Ichilov Hospital in Tel Aviv had found positive results for a cure for COVID-19. A cure! Professor Nadir Arber from the hospital’s integrated cancer prevention center tested a drug he had developed on patients with moderate and severe disease who suffered from the virus, with a 95% positive result. Of the 30 patients who received the drug, 29 showed significant improvement within two days. They could be released from the hospital three to five days later.
According to Professor Arber, the effective drug called EXO-CD24 is inexpensive and only needs to be administered once a day for five days. Where is this drug today? Why was the study swept under the rug?
Another example is the effectiveness of a drug called Allocetra. It was given to 21 critically-ill patients with underlying diseases at Jerusalem’s Hadassah Medical Center. According to the doctors there, 19 patients recovered within six days and were discharged from the hospital after an average of eight days. The drug was developed by Professor Dror Mevorach, director of the Research Center for Rheumatology and Internal Medicine, to treat overactive immune systems that cause cytokines to be released.
Why is there no public discourse regarding this treatment? Certainly, critics could note that these internal studies, which were initially carried out in relatively small framework conditions, are not representative. But the initial success should at least speak for itself. Why have no large-scale studies been commissioned, why does success seem to have been nipped in the bud?
The drug Regeneron, which has now been acquired by the Israeli health authorities, has already received emergency approval from the FDA. It has been shown to reduce hospitalization. It consists of a cocktail of two monoclonal antibodies and was even recommended by the American coronavirus czar Anthony Fauci. “Clinical studies have shown that early treatment with anti-SARS-CoV-2 monoclonal antibodies can reduce the risk of COVID-19 hospitalization or death by 70 to 85 percent,” said Fauci.
Quite a few here are meanwhile wondering why drugs developed in Israel or found to be effective are for some reason not part of the overall strategy to defeat COVID. Is it because they’re just too inexpensive? Why is the much more expensive REGEN-COV now approved? One gets the impression that it is all about profit. And that on the back of people’s health.